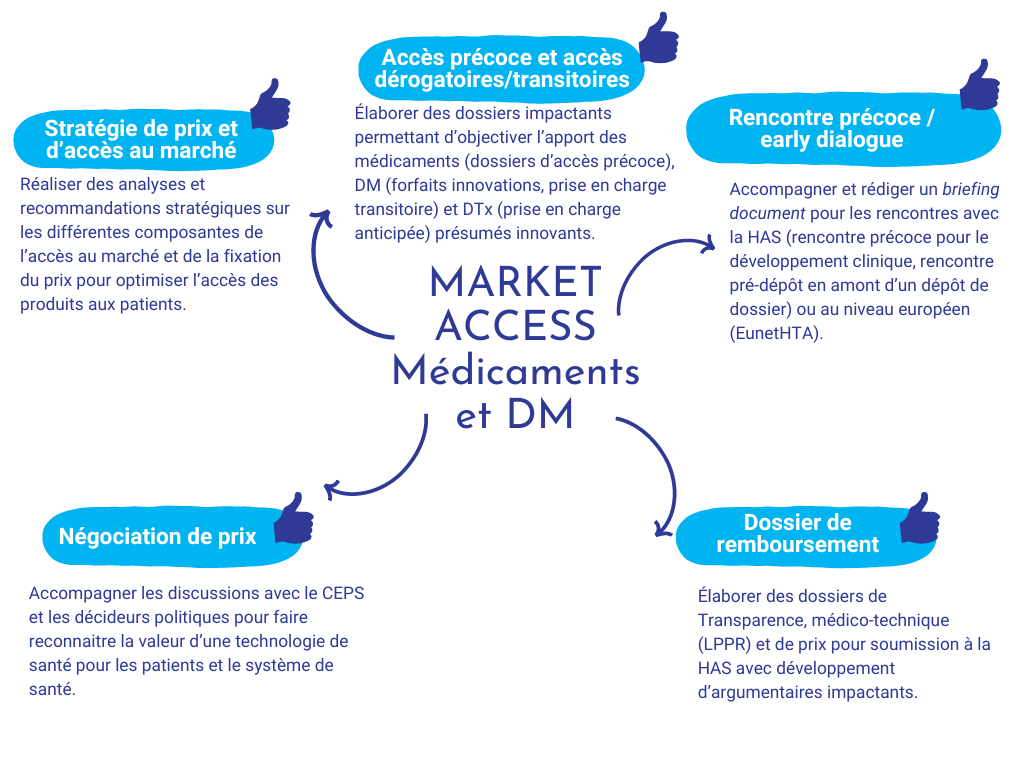
Market Access
Allow faster access to healthcare products for patients
Promoting innovation while taking into account the budgetary impact on the community
Market Access : essential step to provide innovative healthcare solutions to patients

Pharmaceutical industry
Medical Device

Biotech
E-health start up
Our sectoral expertise
Over 20 years’ experience in the evaluation, pricing and market access of healthcare products, in France and internationally
Experienced teams with backgrounds in consultancy, pharmaceutical companies and institutions (ANSM, HAS, CEPS, etc.)
The ability to complement technical expertise with a more general approach to the healthcare system and the levers to be leveraged to speed up the delivery of products to patients.
Recognised expertise in pharmaceuticals, medical technologies and digital services and innovations.
A presence in the main markets of the European Union and North America, enabling us to establish a coordinated global access strategy.
In-depth knowledge of the various routes to market and of changes in evaluation criteria for drugs, medical devices, in vitro diagnostics and digital therapies, particularly as a result of our partnership with SGE Consulting.
Our scopes of intervention